Average book weight 1441 14392 1440 05g 2. The abstract is worth 20 points.
INTRODUCTION The Ideal Gas Law is the equation of state of an ideal gas.

. Also from Avogadros Law we can conclude that the pressure of a gas depends on both volume and temperature but not composition of the gas or whether the gases are all the same orshow more content. Therefore and which gives an isothermal curve so the curve showing the relation between pressure and volume of a given mass of gas when the. To determine the relationship between the pressure and volume of a gas at constant temperature.
There are several ways to verify the law. This is the link of the Boyles Lab. For a fixed amount of gas kept at constant temp the product of the.
2 See answers Advertisement Advertisement frnandagarc05 frnandagarc05 Answer. To Verify Boyles Law Experimentally. Observations on the laws of gases Gasesà targets for the lab.
This is also known as Boyles Law which can be dated back to 1662. AAAAA To gain an experimental apreciaÃà o hurry to barómetros AAAAA To use a tube manómetro à SACU to study relaÃÃμes fundamental between the rush volume and gas temperature of DefiniÃÃμes. Boyles Law_Essay1_Propane Pressure and Volume.
Boyle S Law Lab. BOYLES LAW OBJECTIVE To determine the relationship between pressure and volume of an ideal gas. This is the student online help DATA file for the Boyles Law Lab.
Laboratory Report on Boyles Law law physics 1433 may 2018 law objectives to study law at room temperature and demonstrate that the pressure of fixed mass of. References fTHERMODYNAMICS LAB REPORT TITL E. IB Chemistry - Boyles Law Lab Report.
The data sheet is worth 30 pts. In addition to data this document contains the revisions to the usual procedure to help you adjust for online. This essay needs to include.
1- Calculations -Calculationsformulae shown Figure number and caption provided 2- Discussion -Detailed analysis commentary on results and errors Annotated diagrams with figure number and caption provided where appropriate 3- Conclusion. Advertisement dinzool dinzool Answer. To compare the experimental results with theoretical results.
Boyles Law lab report help. As the pressure of the gas increased the volume of the gas decreased. APPARATUS Pressure sensor syringe and computer.
You should open both this document and the regular procedure document to pick up all the changes. Ideal gas is the gas which composed by many moving molecule and has an elastic collision between the particles. The purpose of this experiment was to understand Boyles LawIn the experiment the pressure in the system under constant temperature and mass was used to confirm if the laws are trueBoyles law relates pressure and volume while all other factors are consistent and states.
The total pressure on the piston equals the pressure from the books plus the atmospheric pressure 1034gcm it does not have uncertainty because it was given. Connect the open end of the syringe to the pressure sensor. Students will investigate Boyles law using syringes gas pressure sensors and labquest digital data collection units.
This experiment was meant to explore the relationship between pressure and gasses described by Boyles Law. In fact Boyles law refers to when at a constant temperature and with a fixed quantity of gas pressure is inversely proportional to volume. The Ideal Gas Law is the equation of state of an ideal gas.
What is a homogeneous solution. Of book Volume 0. The article covers a standard laboratory method to verify the law by studying the relation.
To determine the relationship between pressure and volume of an ideal gas. Essay Sample Check Writing Quality. It relates pressure and volume of gas keeping other parameters amount of gas and temperature constant.
Ideal gas is the gas which composed by many moving molecule and has an elastic collision between the particles. Boyles Law_Essay2_Pressure and Volume Graph 2. Both the answer and the plot are each worth five points.
Students will investigate Boyles law using syringes gas pressure sensors and labquest digital data collection units. Boyles law The relationship between pressure and volume Raw data 1. The gas tend to act like an ideal gas in high temperature and.
Robert Boyle discovered the inverse relationship between pressure and volume in 1662. Boyles law is a famous gas law studied in physics and chemistry. This reveals that pressure is proportional to the inverse of volume.
Boyles Law Lab Report 1. Experiment Number Volume of Gas mL Pressure Atm 1 314 1 2 294 1. All Chemistry ClassesBoyles Law LabName _____ Purpose.
All Chemistry Classes Boyles Law Lab Name _____ Purpose. To determine the relationship between pressure and volume of an ideal gas. Boyle Report Lab Khogr kamal Ibarhim Second Class Production and metallurgy engineering 2018-2017.
This is important because the more pressure an object has the less volume it has. 3- Isochoric change of state gay lussa. To calculate the PV value of.
Students will then complete a formal lab report. For all pairs of data of pressure and volume P V was appoximately the same. ATI COMPREHENSIVE PREDICTOR REVISION GUIDE 2021 500 Correct Questions.
The relationship of pressure to volume for a gas in a rigid container was first described in 1662 by the Irishborn scientist Sir Robert Boyle 16271691 and is known as Boyles La. Lab Report Boyles Law Task 1 PART A. Record the pairs of data for pressure and propane volume in the table below.
Robert Boyle discovered the inverse relationship between pressure and volume in 1662. Check all statements that are true. Advertisement Advertisement New questions in Chemistry.
Note that if T is held constant throughout the experiment then the ideal gas law reduces to Boyles law. Experiment No- 1 Experiment Name -Boyles law lussas law Objective- 1- Demonstrating the laws of state changes in gasses experimentally 2- Isothermal change of state Boyles law. Open the valve and pull the plunger back to set the initial volume of the air in.
Boyles law lab report edgenuity answers. Assignment 3 - To make your life easier. In Excel create a graph of pressure in.
The following is a grading rubric for the abstract. Lab report on Boyles and Guy-Lussacs laws Created Date. THE LAB REPORT Your lab report will consist of your data sheet pg 6 a written abstract the answer to the question below and a plot produced using Excel.
For all pairs of data of pressure and volume P V mr001-1jpg k for the same value k. Students will then complete a formal lab report. Boyles Law is identified through the equation PVk.
Simple Pendulum lab report.
Boyle S Law Lab Flinn Scientific

Solved Experiment 7 Boyle S Law Laboratory Report Show Any Chegg Com

Boyles Law Experiment 1 Xlsx Virtual General Chemistry Laboratory Name Gas Laws Date Experiment 1 Boyle S Law Number Temp Temp Height Pressure Volume Course Hero

Copy Of Gas Law Virtual Experiments Boyle S Law Pdf Virtual General Chemistry Laboratory Https Www Uccs Edu Vgcl Gas Laws Html Name Natalie Course Hero

Lab 10 Boyle S Law Help Boyle S Law Experiment 1 1 2 1 2 3 Record The Pairs Of Data For Pressure Atm And Volume Ml Experiment Course Hero
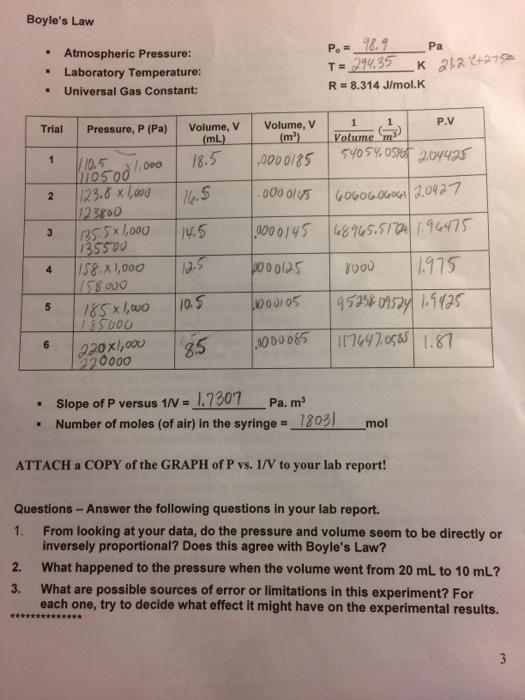
Solved Boyle S Law Pa Atmospheric Pressure Laboratory Chegg Com

Boyle S Law Lab Boyles Law Lab Objective To Determine The Pressure That Is Exerted By The Weight Of The Book Whereas Pressure Equals The Weight Of The Course Hero

Ib Ia Gas Law Experiment Testing Boyles Law Charles Law And Ideal Gas Law International Baccalaureate Chemistry Marked By Teachers Com

Ib Chemistry Charles Law Lab Report International Baccalaureate Chemistry Marked By Teachers Com

Copy Of Lab 2 Boyles Law Student Lab Report Lab Boyle S Law Student Guide Lab Boyles Law Studocu
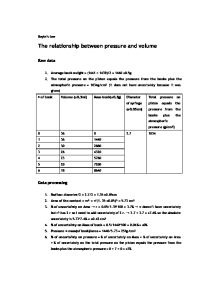
Ib Chemistry Boyle S Law Lab Report International Baccalaureate Chemistry Marked By Teachers Com

Solution Lab 12 Gas Laws Studypool

Boyles Law Lab Report Kamaj Stovall 2 4 Boyle S Law Lab Report Purpose Using Boyle S Law Studocu
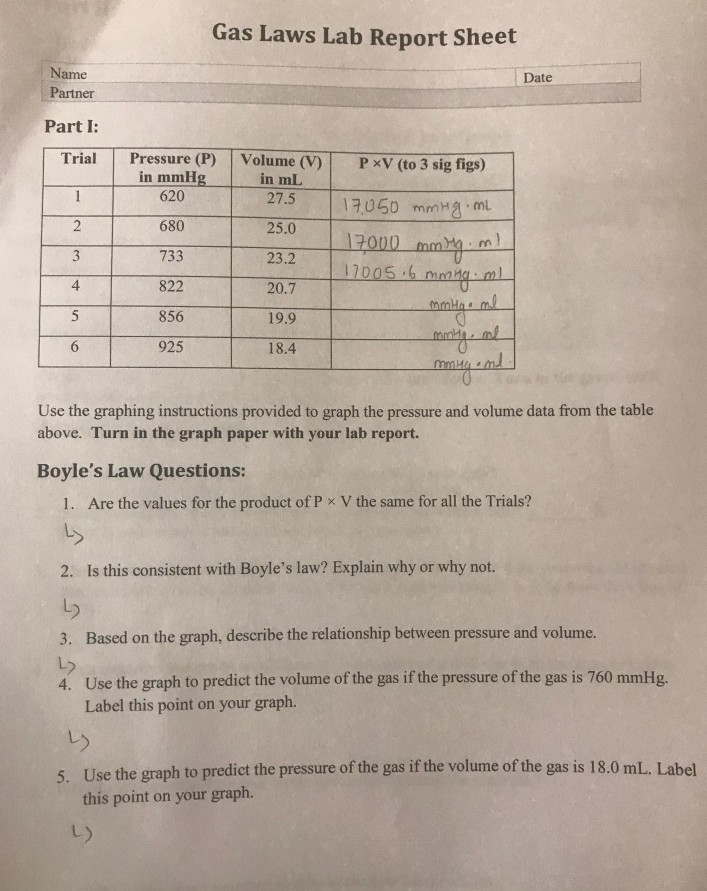
Gas Laws Lab Report Sheet Name Date Partner Part I Chegg Com
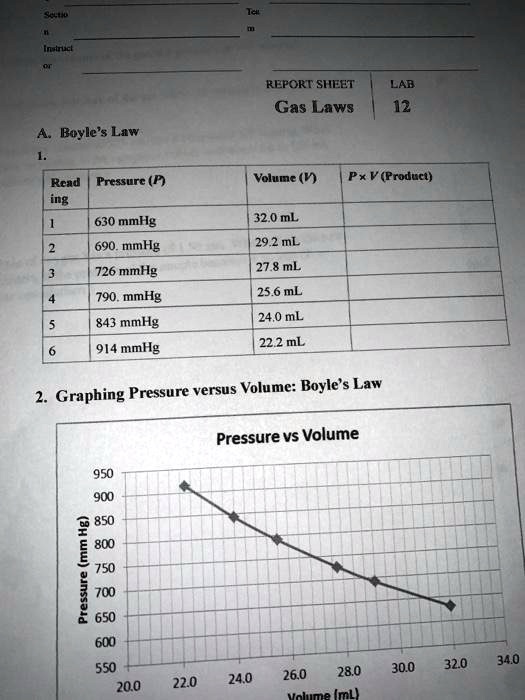
Solved Report Sheet Lab 12 Gas Laws Boyle S Law Read Pressurc P Ing Volume V Pxv Product 630 Mmhg 690 Mmhg 726 Mmhg 790 Mmhg 32 0 Ml 29 2 Ml 27 8 Ml 25 6
Boyle S Law Lab Chem Lab Marlie Chatelain Boyles Law Introduction The Purpose Of This Lab Is To Studocu




